The obesity epidemic is a massive concern for the 21st century. New research shows that the bacteria that live inside our digestive systems could have a large impact on our risk of being obese.
There is a widespread belief, that being overweight or obese is a question of failing willpower, fuelled in no small part by food, fitness and beauty industries. But if we look at the issue of obesity through a scientific spyglass, a very different picture emerges.
Research is increasingly showing that a large part of the tendency to become obese is genetically controlled, often in very subtle ways. Genetic variation could mean that some people are more sensitive to the smell or sight of food, or are less able to sense when they are full. These small variations can play havoc in our modern environment, where calories are readily available and inactive lifestyles are common.
 But it’s not just our own genes that we should be worried about. In terms of processing food, humans are hardly self-sufficient. Our guts are the home of trillions of bacteria that help to break down foodstuffs that our own cells cannot cope with. Together the genes expressed by these intestinal comrades outnumber our own by thousands of times, and yet we are still largely in the dark what they do.
But it’s not just our own genes that we should be worried about. In terms of processing food, humans are hardly self-sufficient. Our guts are the home of trillions of bacteria that help to break down foodstuffs that our own cells cannot cope with. Together the genes expressed by these intestinal comrades outnumber our own by thousands of times, and yet we are still largely in the dark what they do.
Over 90% of these bacteria, collectively known as the microbiota, come from just two groups – the Bacteroidetes and the Firmicutes. Now, new research suggests that the proportion of these groups is an important factor in the obesity epidemic.
 Ruth Ley, Peter Turnbaugh, Jeffrey Gordon and colleagues at Washington University first noticed the link between the microbiota and obesity by studying a special strain of fat mice.
Ruth Ley, Peter Turnbaugh, Jeffrey Gordon and colleagues at Washington University first noticed the link between the microbiota and obesity by studying a special strain of fat mice.
These mice lack the hormone leptin, which controls the body’s ‘fat thermostat’. Without it, the mice cannot monitor the amount of fat in their body and quickly become obese through overeating. The team noticed that these mice had 50% fewer Bacteroidetes and 50% more Firmicutes in their bowels than their lean counterparts.
They saw the same thing in humans. The relative proportion of Bacteroidetes increased in obese people as they lost weight through low-fat or low-carbohydrate diets, while the Firmicutes became less abundant.
The link between the microbiota and obesity became even clearer when Gordon looked at a special strain of mice with no microbiota of their own. These intestinal tabula rasas proved to be strongly resistant to the fattening effects of unhealthy diets. After eight weeks on a 40% fat diet, these animals put on less than half as much weight as their normal peers, despite eating the same amount of food.
When the team transplanted the microbiota from fat and lean mice into the germ-free strains, those colonised by microbiota from fat donors packed on far more weight than those paired with lean donors.
To find out why the shifting bacterial balances were affecting body weight, Gordon and co. compared the microbiota of fat and lean mice at a genetic level. Samples from fat mice showed much stronger activation of genes that coded for carbohydrate-destroying enzymes, which break down otherwise indigestible starches and sugars. As a result, these mice were extracting more energy from their food than their lean cousins.
The bacteria were also manipulating the animals’ own genes, triggering biochemical pathways that store fats in the liver and muscles, rather than metabolising them. While these effects are relatively small, Gordon believes that they can lead to very large fluctuations in weight, over the course of months or years.
Obviously, the microbiota are not the whole story behind the obesity epidemic. We now need to understand how they interact with other things that affect our risk of becoming obese, not least of all, our own genes.
And there is much we still don’t know about our life-long passenger, such as how they sense and respond to their host’s condition, how they are passed on, or how they are affected by our diet. By answering these questions, scientists could then assess whether actively shifting our bacterial balances could help to stem the worldwide increase in obesity levels.
Backhed, Manchester, Semenkovich & Gordon. 2006. PNAS 104: 979-984.
Ley, Turnbaugh, Klein & Gordon. 2006. Nature 444: 1022-1023. Turnbaugh, Ley, Mahowald, Magrini, Mardis & Gordon. 2006. Nature 444: 1027-1031.
Technorati Tags: obesity, bacteria, gut bacteria, intestinal flora, Bacteroidetes, Firmicutes, microbiota, causes of obesity, science
Filed under: Bacteria, Genetics, Health & Medicine, Obesity, Science & society | 5 Comments »
![]() Evolution can sometimes be seen as a futile contest. Throughout the natural world, pairs of species are locked in an evolutionary arms race where both competitors must continuously evolve new adaptations just to avoid ceding ground. Any advantage is temporary as every adaptive move from a predator or parasite is quickly neutralised by a counter-move from its prey or host. Coerced onward by the indifferent force of natural selection, neither side can withdraw from the stalemate.
Evolution can sometimes be seen as a futile contest. Throughout the natural world, pairs of species are locked in an evolutionary arms race where both competitors must continuously evolve new adaptations just to avoid ceding ground. Any advantage is temporary as every adaptive move from a predator or parasite is quickly neutralised by a counter-move from its prey or host. Coerced onward by the indifferent force of natural selection, neither side can withdraw from the stalemate. These patterns of evolution are known as Red Queen dynamics, after the character in Lewis Carroll’s Through the Looking Glass who said to Alice, “It takes all the running you can do, to keep in the same place.”
These patterns of evolution are known as Red Queen dynamics, after the character in Lewis Carroll’s Through the Looking Glass who said to Alice, “It takes all the running you can do, to keep in the same place.”






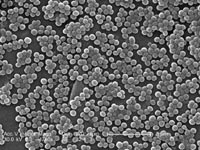 I’ve written an article for the Economist about a new strain of the antibiotic-resistant “superbug” MRSA (methicillin-resistant Staphylococcus aureus) that infects large numbers of farm pigs and can jump into humans.
I’ve written an article for the Economist about a new strain of the antibiotic-resistant “superbug” MRSA (methicillin-resistant Staphylococcus aureus) that infects large numbers of farm pigs and can jump into humans.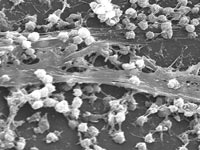 Bacteria may not strike you as expert co-operators but at high concentrations, they pull together to build microscopic ‘cities’ called
Bacteria may not strike you as expert co-operators but at high concentrations, they pull together to build microscopic ‘cities’ called 
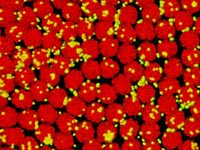



 In a previous article, I described how a micro-organism called Hatena provides us with a snapshot of a key event in evolution. Hatena swallows an alga which becomes an integrated part of its body. Millions of years ago, the ancestors of complex cells did the same thing, taking in bacteria and merging with them to form a single creature.
In a previous article, I described how a micro-organism called Hatena provides us with a snapshot of a key event in evolution. Hatena swallows an alga which becomes an integrated part of its body. Millions of years ago, the ancestors of complex cells did the same thing, taking in bacteria and merging with them to form a single creature.



